Explorer
Will we get nod for vaccine's emergency use? DCGI press conference at 11 am
The Subject Expert Committee of Central Drug Standard Control Organization on Saturday recommended Bharat Biotech's 'Covaxin' for emergency use in India. The final decision on its approval will, however, be taken by the Drug Controller General of India (DCGI).
DCGI will conduct a press conference today.
Notably, the panel recommended emergency licensure for the Serum Institute of India-manufactured 'Covishield'. It becomes the first vaccine to secure recommendation for emergency use in India. The nod of the DCGI is, however, awaited on the recommendation.
DCGI will conduct a press conference today.
Notably, the panel recommended emergency licensure for the Serum Institute of India-manufactured 'Covishield'. It becomes the first vaccine to secure recommendation for emergency use in India. The nod of the DCGI is, however, awaited on the recommendation.
India
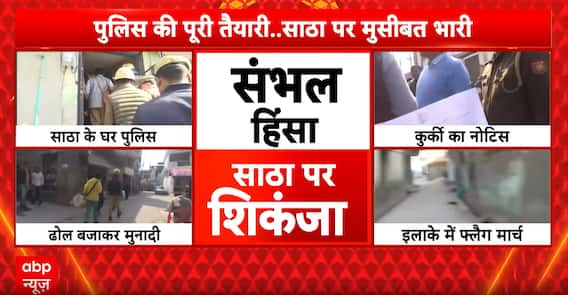
Breaking News: Court Orders Seizure of Shariq Satha’s Assets in Sambhal, Police Flag March Enforced

Breaking News: Trainer Aircraft of Indian Army Crashes in Prayagraj, Rescue Teams at Site

Breaking News: Eyewitness Accounts Reveal Disorder and Alleged Misconduct at Sangam Bath

Breaking News: Noida Engineer Yuvraj’s Death Raises Questions on Police and System Failures

Breaking News: Disciples Allege Disrespect at Magh Mela, Prayagraj Authority Under Fire
View More