Yearender 2022: Corbevax, Covovax, Covishield – Covid-19 Vaccines Used In India And How They Work
Covid-19 vaccination in India: The Covid-19 vaccines currently being used in India are Covishield, Covaxin, Corbevax, Covovax & Sputnik V. Bharat Biotech's nasal vaccine iNCOVACC is also rolled out.

Covid-19 cases are once again on the rise in several regions of the world, including China, Japan, India, United States, South Korea, Brazil, Germany and France. Over 71 per cent of the world's population has received at least one dose of a Covid-19 vaccine. India's Covid-19 vaccination drive began on January 16, 2021. Over 220 crore vaccine doses have been administered in India so far.
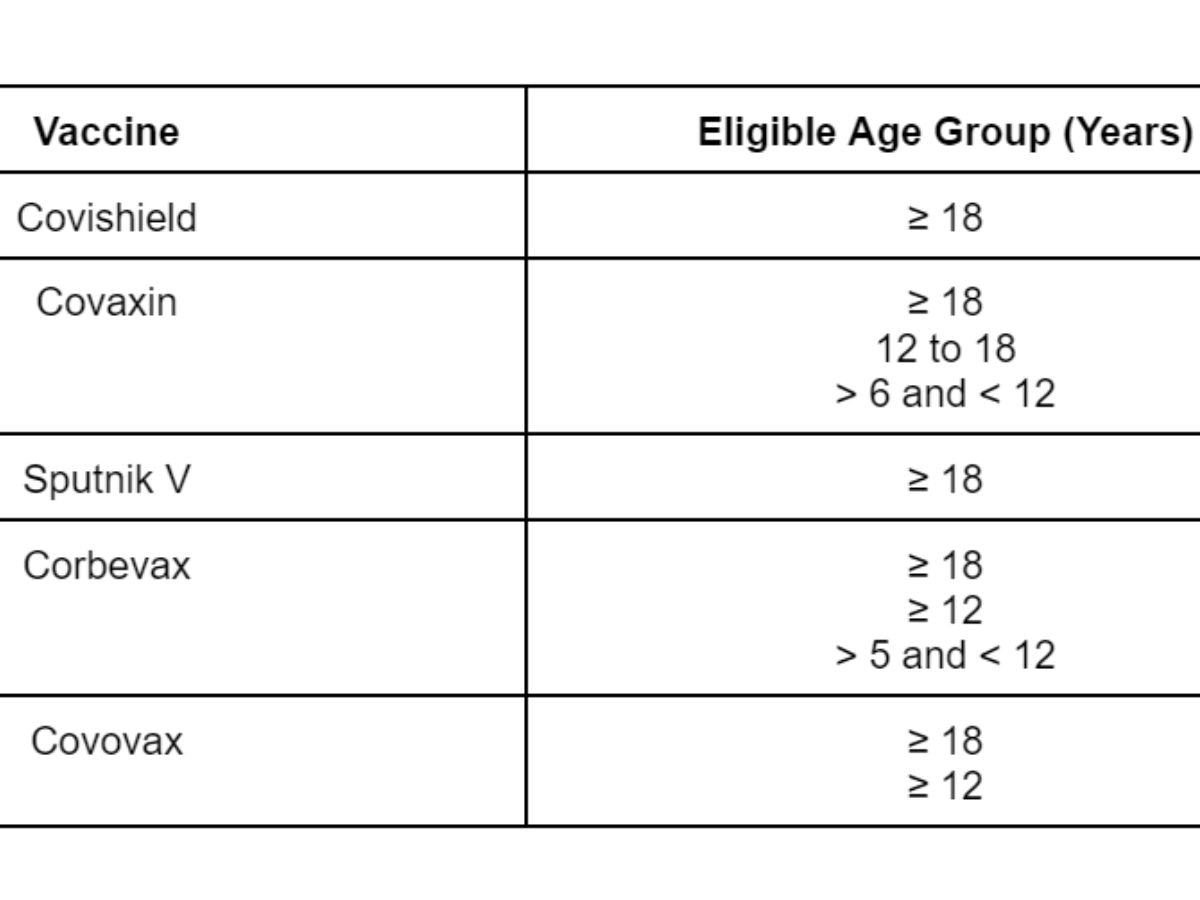
Covid-19 vaccines approved for use in India
Initially, the Oxford-AstraZeneca vaccine, manufactured under licence by the Pune-based pharmaceutical firm Serum Institute of India (SII) under the trade name Covishield, and Bharat Biotech's Covaxin were approved for use in people aged 18 years and above.
Subsequently, other vaccines including Sputnik V, Corbevax and Covovax were approved for emergency use.
Covaxin, India's first indigenously developed Covid-19 vaccine, was approved for emergency use in children belonging to the age group of 12 to 18 years on December 24, 2021. The vaccine was approved for emergency use in children aged above six years and below 12 years on April 26, 2022.
Dr Reddy's Laboratories, which had the distribution rights for Sputnik V in India, was importing the vaccine. In July 2021, Panacea Biotec was allowed to manufacture, distribute and sell Sputnik V vaccines in India. Sputnik V has been developed by the Gamaleya Research Institute of Epidemiology and Microbiology, Russia.
The Moderna Covid-19 vaccine, sold under the brand name Spikevax, was approved for emergency use in India on June 29, 2021. However, later that year, it was decided that the Indian government will not buy Covid-19 vaccines from Pfizer and Moderna.
The Janssen Covid-19 vaccine, which is sold under the brand name Jcovden, was approved for emergency use in India on August 7, 2021. Hyderabad-based pharmaceutical firm Biological E has received approval to manufacture the Janssen Covid-19 vaccine in India. However, the vaccine has not yet been used in the country. If the vaccine becomes available for use in India, it can be administered as a single dose to people aged 18 years and above.
Cadila Healthcare's ZyCoV-D was approved for emergency use in India on August 20, 2021. The world's first DNA-plasmid-based vaccines ZyCoV-D was approved for emergency use in children aged 12 years and above on April 26, 2022. The vaccine has not yet been administered in India.
Covovax, which is manufactured by the SII using technology transfer from Novavax, was approved for emergency use in adults on December 28, 2021. On March 8, 2022, the vaccine was approved for emergency use in children aged 12 years and above.
Biological E's Corbevax was approved for emergency use in adults on December 28, 2021. It was approved for emergency use in children aged 12 years and above on February 21, 2021. The vaccine was approved for inoculation under emergency situations in children belonging to the age group of five to 12 years, on April 26, 2022.
Sputnik Light, a single dose Covid-19 vaccine developed by the Gamaleya Research Institute of Epidemiology and Microbiology, Russia, was approved for emergency use on February 6, 2022.
The HGCO-19 vaccine, also known as Gemcovac or GEMCOVAC-19, and developed by Pune-based Gennova Biopharmaceuticals and Seattle-based HDT Bio, was approved for emergency use in adults on June 28, 2022, according to the Central Drugs Standard Control Organisation (CDSCO).
On September 9, 2022, Bharat Biotech's iNCOVACC, the world's first nasal Covid-19 vaccine, was approved for emergency use in adults. On November 28, 2022, Bharat Biotech announced that the vaccine has been approved for restricted emergency use as heterologous booster doses in adults. The vaccine can now be administered in people aged 18 years and above as primary doses as well as heterologous booster doses. The latter means that a person can receive a different vaccine from the one that was used for the primary dose series.
Following is the list of vaccines being administered in India and who can use them:
How different Covid-19 vaccines work
The Covid-19 vaccines being used in India are of different types. Covishield and Sputnik V are viral vector vaccines, Covaxin is an inactivated vaccine, Covovax is a nanoparticle-based vaccine, Corbevax is a receptor-binding domain protein subunit vaccine, and iNCOVACC is a nasal vaccine.
How Covishield works

Covishield is a recombinant, replication-deficient chimpanzee adenovirus vector vaccine encoding the spike glycoprotein of SARS-CoV-2, according to the SII. A replication-deficient vaccine is one in which each viral particle used as a vector is capable of infecting only a single cell, and can utilise its genetic material only once to induce host cell immune responses. In other words, these viruses are unable to perform the functions necessary for viral genome replication. Covishield uses a chimpanzee adenovirus as the viral vector, and encodes the spike protein of SARS-CoV-2.
According to Mayo Clinic, a vector vaccine is one in which the genetic material from SARS-CoV-2 is placed in a modified version of a different virus, which is known as the viral vector. The viral vector, upon entering the host cells, delivers genetic material from SARS-CoV-2 to the cells, giving them instructions to make copies of the spike protein.
The cells display the spike proteins on their surfaces, following which the immune system responds by creating antibodies and defensive white blood cells.
If one becomes infected with SARS-CoV-2 later, the antibodies will fight the virus. The genetic material delivered as part of viral vector vaccines does not become a part of one's DNA.
How Covaxin works

Whole-Virion Inactivated SARS-CoV-2 Vaccine, or BBV152, is India's first indigenous, whole-virion, inactivated vaccine developed by Bharat Biotech in collaboration with the Indian Medical Research Council (ICMR) and the National Institute of Virology (NIV) for the treatment of Covid-19. BBV152 is the research name for Covaxin.
It is Vero cell derived, which means it is obtained from vero cells from the kidney of an African green monkey. The fact that it is an inactivated whole virion vaccine means that it makes use of the entire pathogenic virus whose genetic material has been destroyed by heat, chemicals or radiation, so that they cause no disease, but provide a good immune response.
An inactivated vaccine is one which consists of the entire pathogenic virus killed or modified in a way such that it cannot replicate or cause disease.BBV152 is formulated with a novel Algel+IMDG adjuvant, and must be administered in two doses, with a gap of 28 days between them.
How Corbevax works
Corbevax is India’s first indigenously developed receptor-binding domain (RBD) protein subunit vaccine against Covid-19. The RBD is a part of the spike protein of SARS-CoV-2. The virus uses the spike protein to attach itself to host cells.
What Is An RBD Protein Sub-Unit Vaccine?
Subunit vaccines, also called acellular vaccines, contain purified pieces of a pathogen, which have been specifically selected for their ability to stimulate immune cells, according to an article published by Gavi, the Vaccine Alliance — a public-private global health partnership that helps vaccinate almost half of the world’s children against deadly and debilitating infectious diseases.
Subunit vaccines are of different types, and are considered safe because the purified fragments are incapable of causing disease. They include only the parts of a virus that best stimulate one’s immune system, according to a Mayo Clinic article.
Protein subunit vaccines contain specific isolated proteins from viral or bacterial pathogens, and are being developed against SARS-CoV-2. Hepatitis B vaccines are other examples of protein subunit vaccines. An RBD protein subunit vaccine against Covid-19 makes use of a specific part of SARS-CoV-2 — the spike protein. It is a recombinant vaccine, which means it is manufactured using living organisms such as yeast cells.
RBD protein subunit vaccines are suitable for people with compromised immune systems, consist of no live components, as a result of which there is no risk of the vaccine triggering disease, and are relatively stable. However, they are more complex to manufacture, compared to other Covid-19 vaccines, and adjuvants and booster shots may be required after receiving the primary vaccination doses.
How Does An RBD Protein Subunit Vaccine Work?
An RBD protein subunit vaccine is one which induces protective immunity by targeting the RBD of the S protein of SARS-CoV-2, which is a harmless protein. The vaccine comprises residues of the RBD protein, according to a study published in the journal, Nature.
The residues of the RBD protein used in the vaccine are carefully studied to identify which combinations of the molecules are likely to produce a strong and effective immune response, according to the Gavi article. The body develops an immune response against the injected fragments of the spike protein.
The immune system recognises the S proteins, and creates antibodies and defensive white blood cells. If the person becomes infected with the Covid-19 virus in future, the antibodies will fight the virus.
An RBD protein subunit vaccine, like Corbebax, helps the immune system recognise the S proteins, and create antibodies.
How Covovax works
SII’s Covovax is a nanoparticle-based vaccine. It is being manufactured by technology transfer from Novavax. Covovax is approved by the European Medicines Agency for conditional marketing authorisation, and has been granted emergency use listing by the World Health Organization (WHO).
What Is A Nanoparticle-Based Vaccine?
A nanoparticle-based vaccine is one in which the receptor-binding domain (RBD), which is a part of the spike protein of SARS-CoV-2, is attached to a protein designed to form nanometre-sized protein particles, or nanoparticles, according to a study by the US National Institutes of Health (NIH), which was published in the journal, Nature. SARS-CoV-2 attaches itself to cells using the spike protein.
These nanoparticles could be composed of lipids, metal and non-metal inorganics, several polymers, and virus-like particles which have been tested for research, according to a study published by the National Center for Biotechnology Information (NCBI), NIH. Virus-like-particles (VLP) are self-assembling nanoparticles lacking infectious nucleic acid.
How Does A Nanoparticle-Based Vaccine Work?
Nanoparticles help improve vaccine efficacy, by targeting desired antigen-presenting cells to improve immunisation strategies. They protect the antigen (foreign particle) from early proteolytic degradation (degradation of protein by hydrolytic enzymes), control antigen release, and facilitate antigen uptake.
Current vaccines cause cells in the body to make a version of the spike protein to elicit an immune response. Putting multiple copies of the RBD on nanoparticles enhances immune response, recent studies have found. The NIH researchers tested nanoparticle-based vaccines in monkeys and found that most of the monkeys had no virus in their lower respiratory tracts two days after exposure.
Nanoparticle-based vaccines contain a strand of genetic code that provides instructions for building a version of the spike protein, according to an article by the University of Pittsburgh Medical Center, US. Host cells build the protein when they see the genetic code.
The immune system begins building antibodies against the protein, which help the immune system fight the coronavirus. The body builds up an army of antibodies, which can fight off a Covid-19 infection before it causes disease.
Since mRNA is very fragile on its own, it would degrade in the body if it is injected simply as a strand. Hence, the genetic material is protected with a nanoparticle, which preserves the mRNA long enough to carry it into the body’s cells so that they can begin making the proteins.
The use of nanoparticles in vaccine formulations allows not only improved antigen stability and immunogenicity, but also targeted delivery and slow release, according to an article published in the journal, Elsevier.
The Covovax vaccine, which is based on Novavax, will work by teaching the immune system to make antibodies to the spike protein on SARS-CoV-2.
How iNCOVACC works
Bharat Biotech's intranasal Covid-19 vaccine is a recombinant replication deficient adenovirus vectored vaccine. This means that critical portions of the genome of SARS-CoV-2 have been removed, such that the viral vector can no longer replicate. The fact that it is a recombinant vaccine means that it has been manufactured using bacterial or yeast cells or parts of a virus to introduce viral proteins into the host. An adenovirus vectored vaccine means that a modified version of an adenovirus has been used which can enter human cells but not replicate inside.
Adenovirus-vectored vaccines are the vaccines in which adenoviruses are used as vectors for delivering a particular antigen into the body of a host, where the cells will read the foreign particle, and create antibodies against it.
The phase I, II and III clinical trials yielded successful results. The vaccine will allow intranasal delivery through nasal drops, a system designed and developed to be cost-effective in low- and middle-income countries.
Bharat Biotech's iNCOVACC has the dual benefit of enabling faster development of variant-specific vaccines and easy nasal delivery. This will allow mass immunisation and protect people from emerging variants of concern, according to the statement. The launch dates, price and availability of the vaccine will be announced soon.
The optimum temperature range for storage and distribution of iNCOVACC is two to eight degrees Celsius.
Working of iNCOVACC
An intranasal vaccine stimulates a broad immune response by producing a wide range of antibodies including neutralising Immunoglobulin G (IgG) and mucosal Immunoglobulin A (IgA). The vaccine also initiates T cell responses.
According to the official website of Bharat Biotech, immune responses in the nasal mucosa, which is the site of infection, are essential to block the infection and stop the transmission of Covid-19.
When the iNCOVACC vaccine is administered into a person, the immune cells in the body will express the stabilised spike protein. As a result, the body will produce antibodies against the spike protein of SARS-CoV-2.
If a person is naturally exposed to SARS-CoV-2, the immune system will prevent Covid-19 infection in both upper and lower respiratory tracts due to the presence of antibodies. Thus, iNCOVACC has the potential to block SARS-CoV-2 and prevent the transmission of Covid-19.
What Are The Advantages Of iNCOVACC?
Intranasal SARS-CoV-2 vaccines are likely to prevent infection and transmission, and also prevent disease.
According to Bharat Biotech, the advantages of iNCOVACC are that it is non-invasive and needle-free, can be administered easily, is ideally suited for children and adults, and has scalable manufacturing.
The use of this vaccine will eliminate needle-associated risks such as injuries and infections. Also, the nasal mucosa has an organised immune system because of which the nasal route serves as one of the best regions for vaccination.
Where Does iNCOVACC Produce Antibodies?
Since iNCOVACC is an intranasal vaccine, it has the potential to produce local antibodies in the upper respiratory tract, according to the statement. This can potentially reduce infection and transmission of SARS-CoV-2.
On December 27, 2022, Bharat Biotech announced that iNCOVACC, which is now available on CoWIN portal, is priced at Rs 800 for private markets and at Rs 325 for government supplies.
Check out below Health Tools-
Calculate Your Body Mass Index ( BMI )