Centre Eases Export Policy Over Remdesivir Injection, Drug Now In 'Restricted' Category
In a notification issued on June 14, the Ministry of Commerce and Industry said: "Central Government hereby amends the notification dated 11.04.2021 related to the export of injection Remdesivir and Remdesivir API."
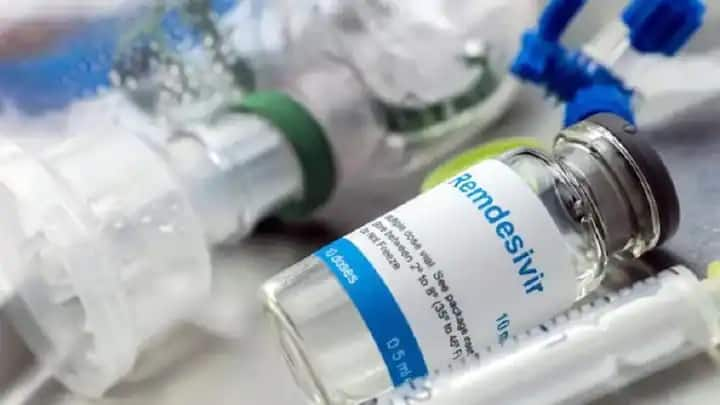
Centre on Monday lifted the two-month-long ban on the export of Remdesivir drug, allowing overseas sales without “special” authorization.
The Government of India on June 14 amended the export policy for injection Remdesivir and Remdesivir Active Pharmaceutical Ingredients (API), permitting the export of the drug used in COVID-19 treatment with immediate effect.
In a notification issued on June 14, the Ministry of Commerce and Industry said: "Central Government hereby amends the notification dated 11.04.2021 related to the export of injection Remdesivir and Remdesivir API."
Thanks to the new revision the export of Remdesivir injections and APIs against the Advance Authorization shall no longer require a separate export authorization or permission.
Considering the pandemic’s second wave, the export of Remdesivir injection was halted back in April. The cases of Covid-19 were on a rise back then, and so was the demand for the drug. Thus, as a precautionary measure, the drug was placed under the “prohibited’ category. The new amendment has brought the drug into the "restricted” category.
"Chapter 4 of FTP/HBP shall not require a separate export authorisation or permission,", said the commerce ministry's director-general of foreign trade.
Related Video
Breaking News: Software Engineer Yuvraj Dies in Water-Filled Pit, Systemic Negligence Questioned






































