Explorer
Advertisement
Will abide by standard protocol: SII to DGCI's notice on Covid vaccine's trial
"The Serum Institute of India Pvt Ltd, Pune, till now has not informed the Central licensing authority regarding pausing the clinical trial carried out by AstraZeneca in other countries and also not submitted casualty analysis of the reported serious adverse event with the investigational vaccine for the continuation of phase 2 and 3 clinical trials of the subject vaccine in the country in light of the safety concerns," the notice By DGCI read.
All Shows
Advertisement
Top Headlines
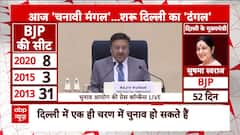
Election 2024
Election 2024
India
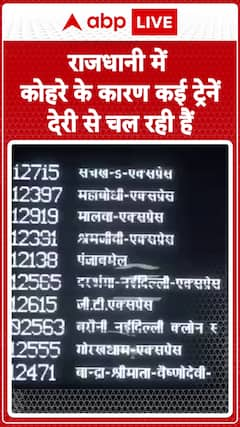
Cities
Advertisement
Advertisement
Trending News

Advertisement